New materials to create more durable and efficient batteries are wanted. Many companies and laboratories are developing fluoride batteries. Today is shown these batteries and theirs promises.
Why fluoride?
Actually, the best commercial batteries available have lithium. However, lithium-ion batteries can catch fire that is hard to extinguish. Already exist cases of electric cars with lithium batteries catching fire in parking lot, click here to see. In addition to that, there are demand for more durable batteries.
Fluorine is the element with highest electronegativity, whose valor is 4.0 and has low atomic mass, whose value is 18.998 a.m.u. It is a very reactive and dangerous element, but the fluoride ion is stable, because your valence layer is complete with 8 electrons.

Fluoride batteries can have 8 or 10 more energy density than lithium batteries. Because metal fluoride can transfer more electrons than metallic lithium oxide. These are the chemical reaction equations of a lithium-ion cell and this cell’s schematics respectively.

Operation of battery
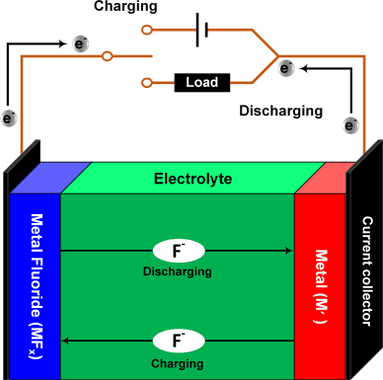
Fluoride battery is negative, because fluoride ions are negative. In opposition of lithium positive ions. The cell has an electrolyte where fluoride ions move in direction to charging or discharging. The metal (M') is anode and metal fluoride (MF_{x}) is cathode. These equations are discharging chemical reactions in cathode and anode electrode respectively, to charging reaction, just change arrow’s direction. Usually, metal M is copper (Cu), in this case, x is equal to 2. Comparing with lithium-ion equations above, can observe fluoride battery moves more electrons. Therefore, has higher energy density.
xe^{-}+MF_{x}\rightarrow M+xF^{-}
xF^{-}+M'\rightarrow M'F_{x}+xe^{-}
The fluoride battery isn’t a new technology, was developed in 1970s, but were impractical until recently. Because solid electrolyte need to stay at 150ºC to fluorine dissolve and make the electrolyte conductor, this temperature is impractical. Recently, through computer simulations to test new materials, researchers discovered a material which can be a liquid electrolyte at room temperature. It is called bis(2,2,2-trifluoroethyl) ether or BTFE, keeps ion stable to go from an electrode to another. In this image, fluoride is the purple ball in electrolyte.

For now, this cell is at research laboratories, still exist other obstacles.
Fluoride batteries for electric cars
As was explained in the post about comparison between electric and combustion vehicles. The main advantage of combustion car is energy density.

If a fluoride battery have 10 times more energy density than lithium-ion, can reach liquid hydrogen but still will be less dense than gasoline and diesel. However, batteries with more energy density will greatly increase autonomy of electric cars, computers, drones and smartphones. For that, companies like Honda invest in the development of these new batteries.



