How do the adhesion mechanisms used by wall climbing robots work? In this post, are shown the three forms of adhesion.
Adhesion mechanisms of wall climbing robots
Pneumatic mechanisms
Some robots use suction cups to fix on walls.
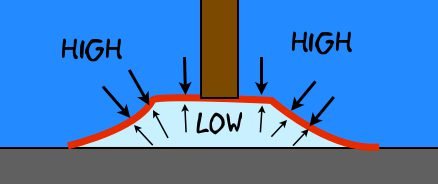
When a suction cup is pressed against the wall, the volume between suction cup and surface decreases and pressure increases. Part of inner air goes out through sides. After the pressure, the suction cup goes back to its original size and the inner pressure (light green) is reduced. Consequently, the external pressure (blue) becomes bigger than inner pressure and the robot are fixed on wall with suction cups.
The suction cups are only useful on smooth surfaces because on irregular surfaces, air goes inside the suction cup, leading to increasing inner pressure. Another pneumatic method is the negative pressure thrust. Consist of using a high speed motor and a propeller to suck the air between wall and robot’s lower part, reducing internal pressure.
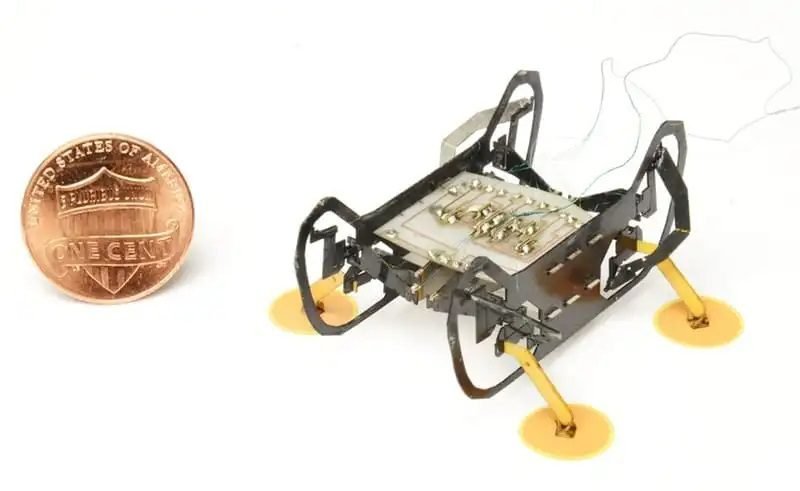
Robots with negative pressure thrust consume more energy to keep in the walls.
Magnets
Some robots use magnets to climb metallic or ferromagnetic walls.
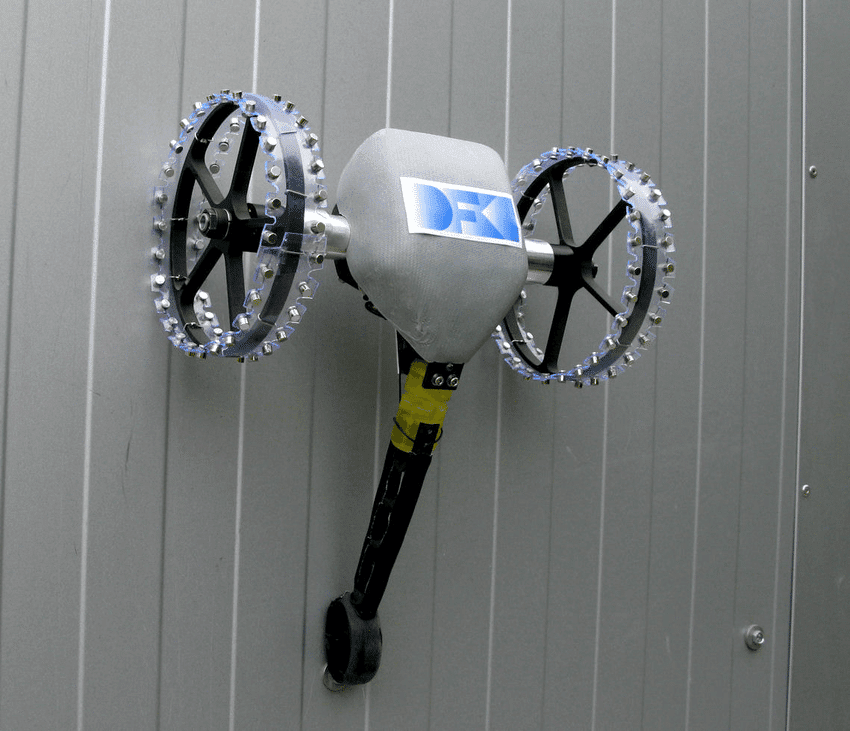
Adhesives with microfibers
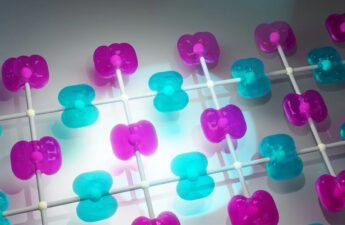
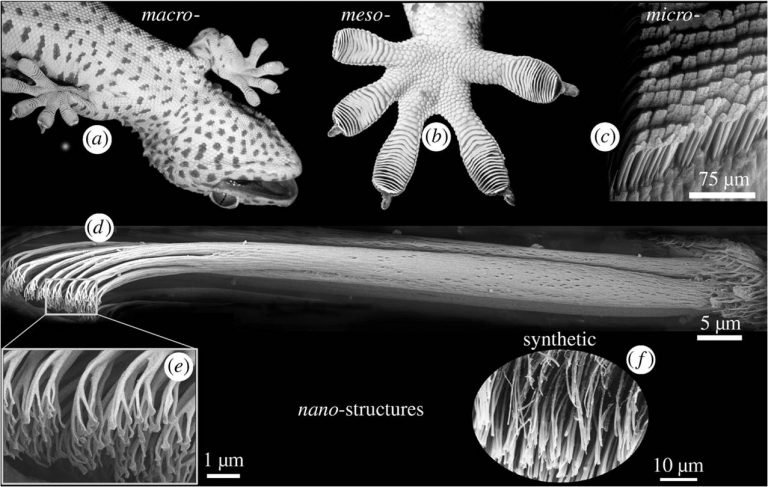
This technology is inspired by nature, some animals like the gecko can climb walls because its paws have millions of filaments that stick on the surface using Van der Waals forces.
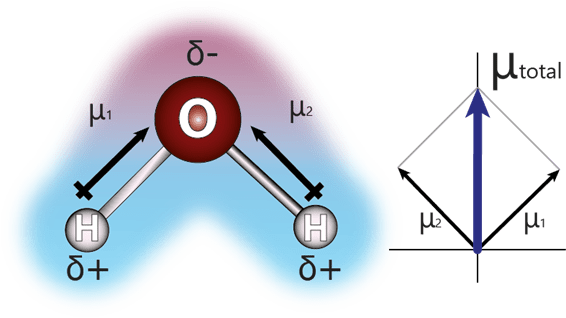
What are Van der Waals forces? They are intermolecular forces of attraction and repulsion caused by electric dipoles. Polar molecules have unequal distribution of electrons, one side has a positive charge and another with a negative charge, forming an electric dipole. The polar molecules have a resultant dipole moment \mu_{total}.
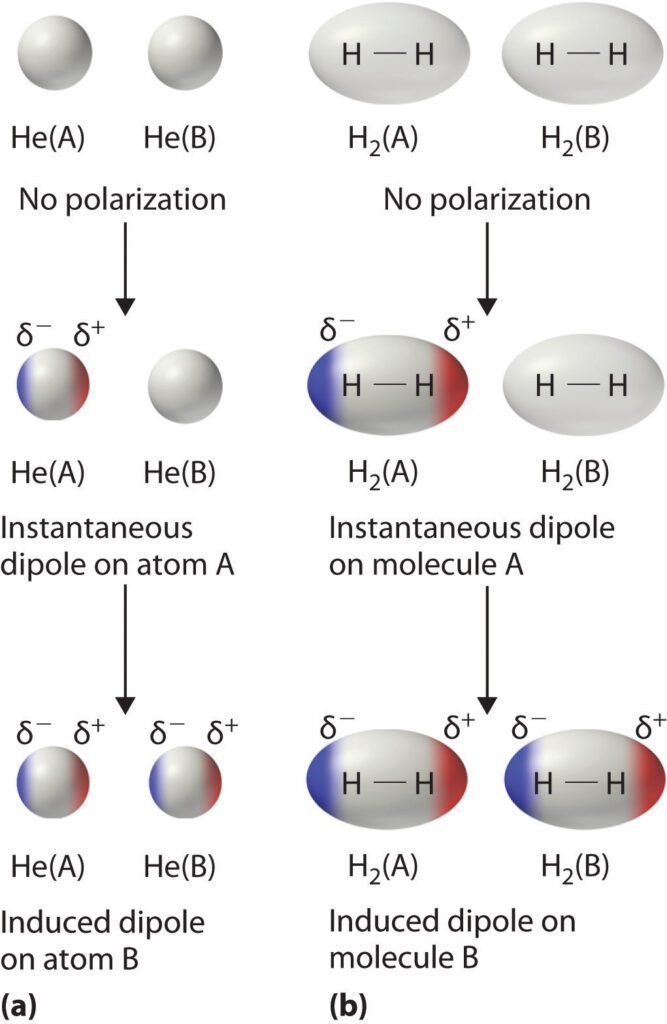
The molecules have fluctuations in the electronic cloud, due to constant motion of electrons. In apolar molecules, these fluctuations can make molecules temporarily polarized. Could electrically induce polarization in neighboring molecules and create attraction electrostatic forces.
When spatulae are in contact with the surface, the former spread, and spatulae’s molecules become dipoles that induce surface molecules to polarization. Creating an electrostatic force between the toes and the wall. Van der Waals force is the weakest of intermolecular forces. But, when added with millions of setae and the spatulae’s thin thickness, which allows a big surface contact area, there’s enough force to keep the gecko on walls or ceilings.