Meeting requests, today’s subject is the operation of pHmeter. An instrument that measures acidity and alkalinity of solutions.
To know what is pH and its importance to environmental balance, click on the following link.
pH: definition and importanceClick here
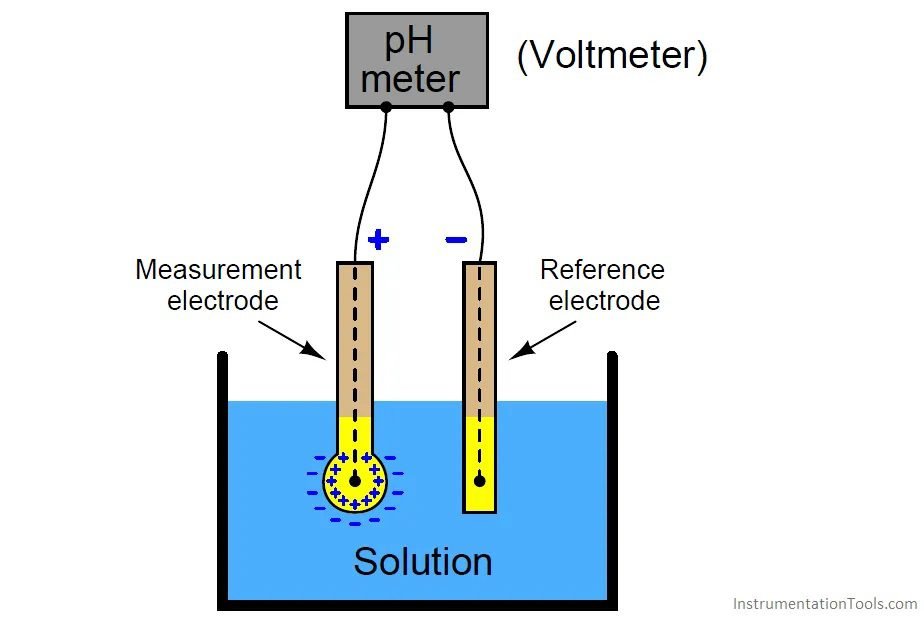
How the pHmeter works?
Two probes are used, one of reference and other for measurement. The measurement probe is made of glass with a small bulb on bottom, which is in contact with the solution to be measured.
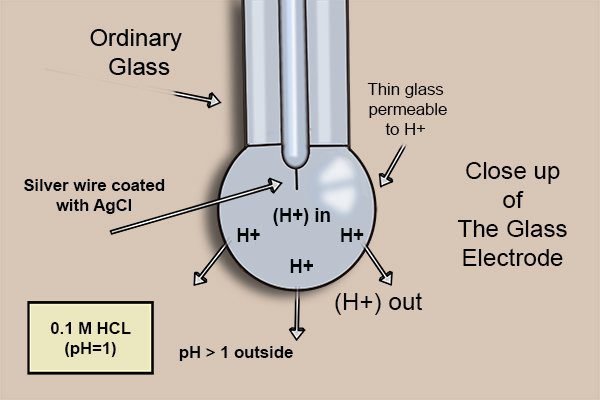
The bulb is made of a thin glass membrane that is permeable to hydrogen ions (H^{+}). Inside the glass tube there’s a silver electrode, coated with silver chloride (AgCl) and a chloride potassium (KCl) solution, whose pH is 7.


When there’s a concentration difference, there’s a ion exchange between the solution and inner and outer gel layers. H^{+} ions of higher concentration solution go to glass membrane, replacing lithium (Li^{+}) or sodium (Na^{+}) and the latter go to gel layer with less positive ion concentration.
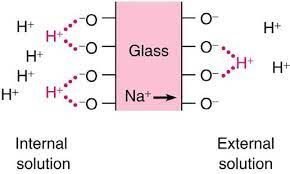
If solution’s pH was 7, there wouldn’t be ion difference concentration inside and outside the probe. Therefore, the difference potential will be 0.
Reference probe
Determine pH measure precision, must have a constant electric potential and low temperature coefficient. Also has a silver electrode with AgCl coating and the same KCl electrolyte. The reference probe is in contact with the measured liquid through a porous junction for KCl diffusion and close the electric circuit.

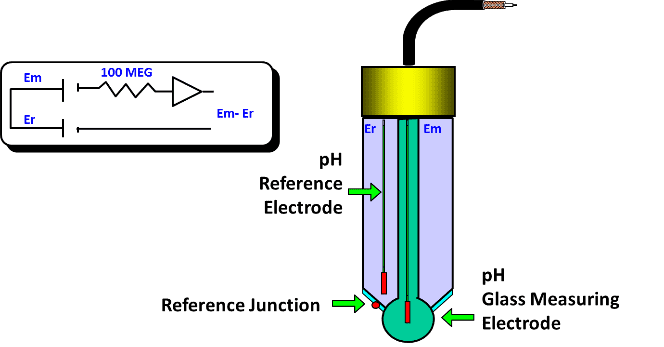
There’s a small electric voltage between the electrodes, which is amplified to pHmeter calculate the pH using Nernst equation and show on display.
Nernst equation
Relates electric voltage V with ion concentration difference in two substances.
V=\frac{RT}{nF}ln(\frac{C_{1}}{C_{2}})
Where:
- R is the gas universal constant, whose value is 8.315 J/mol\cdot K.
- T is temperature in Kelvin.
- n is the number of transferred electrons by ion exchanged.
- F is Faraday constant, whose value is 96485 C/mol (coulombs por mole).
- C_{1} is ion concentration on measured solution, in moles/liter of solution.
- C_{2} is ion concentration on reference solution.
I was asked if a pHmeter made to operate at 50 Hz will work differently on 60 Hz, or vice-versa. Since this instrument’s operation principle don’t depend on grid frequency, there won’t be any alteration.



